-
 [From left] Study authors Prakhar Gupta, Yueze Gao and Harry Anderson holding a model of part of the catenane. Credit: Dr Robert Eichelmann.
[From left] Study authors Prakhar Gupta, Yueze Gao and Harry Anderson holding a model of part of the catenane. Credit: Dr Robert Eichelmann. -
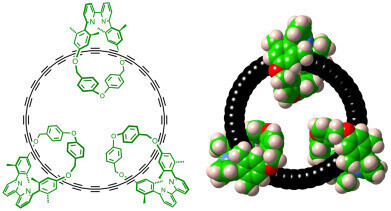 Left: Chemical structure of the cyclo[48]carbon [4]catenane. Right: Space-filling representation. Credit: Harry Anderson.
Left: Chemical structure of the cyclo[48]carbon [4]catenane. Right: Space-filling representation. Credit: Harry Anderson.
Research news
UK research team synthesises stable cyclocarbon allotrope at room temperature; first time since 1990
Aug 14 2025
A research team led by the University of Oxford’s Department of Chemistry has reported the synthesis of an entirely new molecular form of carbon that is stable enough to study in solution at room temperature. The achievement, which has been described as a significant milestone in synthetic carbon chemistry, represents only the second time in history that a novel molecular carbon allotrope has been obtained under normal laboratory conditions. The only previous synthesis was in 1990 when ‘fullerenes’ were made by Wolfgang Krätschmer et al.
The new molecule – cyclo[48]carbon – was created in the form of a [4]catenane – a structure in which the C48 carbon ring is threaded through three other large macrocyclic molecules. This threading serves a protective role, physically preventing reactive species from attacking the delicate cyclocarbon and thereby greatly increasing its stability. The strategy addresses a long-standing challenge in carbon allotrope chemistry, as cyclocarbons have historically been too unstable to isolate and study in condensed phases without extreme conditions.
Before the development in this work, pure carbon rings had previously only been characterised in the gas phase or at cryogenic temperatures between 4 and 10 Kelvin. By contrast, the Oxford-led team has synthesised a cyclocarbon stable in solution at 20°C, with a half-life of 92 hours.
This unprecedented stability was achieved through a combination of design choices: the use of threaded macrocycles to shield the ring, the selection of a relatively large carbon ring to minimise structural strain and the development of mild chemical conditions for the final unmasking step – in which a precursor compound is transformed into the reactive target molecule.
The characterisation of the cyclocarbon catenane drew on a range of analytical techniques, including high-resolution mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy, ultraviolet–visible (UV–vis) absorption spectroscopy and Raman spectroscopy. A particularly striking observation was the detection of a single intense ^13C NMR signal corresponding to all 48 carbon atoms in the ring, each in an identical chemical environment. This finding provides compelling structural evidence for the equivalence and arrangement of the atoms within the cyclocarbon catenane.
“Achieving stable cyclocarbons in [the test tube] at ambient conditions is a fundamental step.
“This will make it easier to study their reactivity and properties under normal laboratory conditions said lead author Dr Yueze Gao of the University of Oxford’s Department of Chemistry.
“This achievement marks the culmination of a long endeavour to synthesise cyclocarbon catenanes, based on the hope that they might be stable enough to study at room temperature,” , added the study’s senior author Professor Harry Andersen, also of Oxford’s Department of Chemistry.
“The original grant proposal was written in 2016, based on preliminary results from 2012 to 2015. It is satisfying to have reached this point, because there were many times when the goal seemed unrealistic and unachievable,” he continued.
“This work would not have been possible without the outstanding facilities for NMR spectroscopy in the Department of Chemistry at Oxford.”
The research brought together expertise from the University of Oxford, the University of Manchester, the University of Bristol, and the Central Laser Facility at the Rutherford Appleton Laboratory.
Beyond its immediate significance for synthetic chemistry, the work opens up the possibility of studying the reactivity, electronic properties and potential applications of cyclocarbons without the constraints of cryogenic or high-vacuum environments. Such insights could have long-term implications for molecular electronics, nanomaterials and carbon-based quantum systems.
For further reading please visit: 10.1126/science.ady6054
Digital Edition
Lab Asia Dec 2025
December 2025
Chromatography Articles- Cutting-edge sample preparation tools help laboratories to stay ahead of the curveMass Spectrometry & Spectroscopy Articles- Unlocking the complexity of metabolomics: Pushi...
View all digital editions
Events
Jan 21 2026 Tokyo, Japan
Jan 28 2026 Tokyo, Japan
Jan 29 2026 New Delhi, India
Feb 07 2026 Boston, MA, USA
Asia Pharma Expo/Asia Lab Expo
Feb 12 2026 Dhaka, Bangladesh


















